Africa Clinical Trials Market Size, Share & Industry Analysis, By Phase (Phase I, Phase II, Phase III, and Phase IV), By Application (Oncology, CNS Disorder, Cardiology, Infectious Disease, Metabolic Disorder, Renal/Nephrology, and Others), and Regional Forecast, 2024-2032
KEY MARKET INSIGHTS
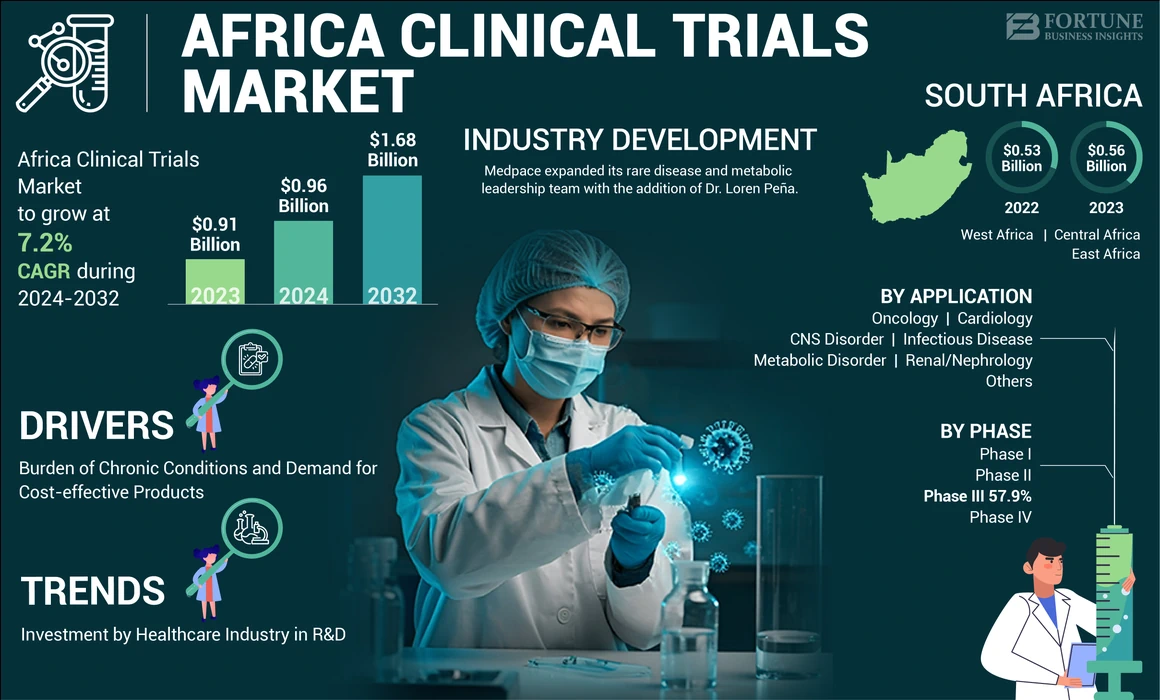
The Africa clinical trials market size was valued at USD 0.91 billion in 2023. it is projected to grow from USD 0.96 billion in 2024 to USD 1.68 billion by 2032, exhibiting a CAGR of 7.2% during the forecast period.
The burden of chronic conditions such as including malaria, HIV/AIDS, tuberculosis, and non-communicable diseases, such as diabetes, cancer, and others, along with the high mortality rates due to such diseases, has been increasing significantly in Africa. This factor has been fueling the demand for effective and novel diagnosis and treatment options to reduce the growing burden of the disease. In order to fulfill this growing demand, pharmaceutical and biotechnology companies have increased their focus on conducting clinical studies for the development and launch of effective therapeutics, thereby fueling the Africa clinical trials market growth.
- For instance, F. Hoffmann-La Roche Ltd initiated a phase II clinical trial in January 2021 to study the safety and efficacy of targeted therapies or immunotherapy as single agents or in rational, specified combinations among patients with locally advanced or metastatic solid tumors. The study is expected to be completed in September 2032. It was conducted at 175 locations, including South Africa.
Furthermore, conducting clinical trials in Africa is comparatively cheaper than in other countries globally, and it also reduces certain challenges, such as a limited patient pool, stringent regulatory policies, and others, compared to the rest of the world.
Moreover, the increasing focus of the government and regulatory bodies on initiatives to enhance the trial programs has also been fueling the market growth.
- For instance, in May 2023, the Africa CDC, along with AUDA-NEPAD, assembled sixty experts from across the region and globally to discuss solutions to strengthen the African clinical trial ecosystem.
During the COVID-19 pandemic in 2020, the market experienced growth in its value. This was due to the increased focus of pharmaceutical and biotechnology companies on R&D to develop effective diagnosis, treatment, and prevention options for COVID-19.
Moreover, in 2021, the market experienced a significant boost in its market value due to the initiation of clinical studies that were put on hold in 2020 because of the sudden outbreak of COVID-19.
AFRICA CLINICAL TRIALS MARKET OVERVIEW
Market Size:
- 2023 Value: USD 0.91 Billion
- 2024 Value: USD 0.96 Billion
- 2032 Forecast Value (with CAGR): USD 1.68 Billion (CAGR of 7.2%)
Market Share:
- Regional Leader: South Africa
- Fastest-Growing Region: East Africa
- End-User Leader: Infectious Disease Segment
Industry Trends:
- Increasing Investment by Healthcare Industry in R&D to Fuel Number of Trials Being Conducted in Region
- Decentralization and Digital Health Adoption
- Regulatory Harmonization Initiatives
Driving Factors:
- Growing burden of chronic conditions such as cancer, diabetes, Ebola, and CVD fueling demand for effective treatment options
- High treatment costs prompting demand for cost-effective therapeutics and local manufacturing to reduce import expenses
- Emphasis on disease-specific trials targeting highly prevalent conditions like malaria, HIV, and Ebola
- Adoption of innovative trial designs including adaptive and remote models to overcome logistical challenges
- Capacity building and training initiatives by organizations like Africa CDC and AUDA-NEPAD to develop skilled personnel and modern infrastructure
- Presence and expansion of CROs focusing on Africa’s genetically diverse population to support global clinical research initiatives
Africa Clinical Trials Market Trends
Increasing Investment by Healthcare Industry in R&D to Fuel Number of Trials Being Conducted in Region
The growing burden of chronic conditions such as infectious diseases has been fueling the demand for effective therapeutics and has been fueling the pharmaceutical companies’ emphasis on effective drug development. Along with this, the increasing demand for inexpensive medicines in African countries has also been fueling the demand for the development of novel and cost-effective therapeutics in the region. This is because the development of medicines locally in Africa could eliminate the import costs that are applicable to products being imported from other parts of the world and would reduce the overall cost of treatment through these therapeutics.
- For instance, as per the data published by the World Economic Forum in March 2023, the demand for medicines in Africa is around USD 18.0 billion. Of this, 61% are imported, 36% are locally produced, and 3% are met by intra-African trade.
Furthermore, many Contract Research Organizations (CROs) are focusing on partnerships and collaborations in order to expand their service offerings in the region.
- In March 2024, Croissance Clinical Research, an India-based CRO, partnered with The African Clinical Research Management Initiative (ACRMi), a Kenya-based CRO, to launch Croissance Clinical Research-Africa (CCR-A). With this launch, both companies aimed to enhance their offerings in diversified therapeutic areas, including infectious disease, and neglected infectious disease.
Therefore, the increasing investment by pharmaceutical companies in new therapeutics development, along with the increasing emphasis of Contract Research Organizations (CROs) on the expansion of their service offerings, has been increasing the number of trials being conducted in the region.
Other Trends:
- Decentralization and Digital Health: Contract Research Organizations (CROs) and healthcare companies have also been focusing on the adoption of digital tools and Decentralized Clinical Trials (DCTs) to reach remote populations and improve trial efficiencies.
- Regulatory Harmonization Initiatives: African regulatory bodies, such as the African Medicines Agency (AMA) and Regional Economic Communities (RECs), have been focusing on creating standardized regulatory frameworks to attract more trials.
Opportunities
- Improving Infrastructure: Recommendations for enhancing infrastructure, with an emphasis on digital health technologies and decentralized trial models, will enhance the clinical study process.
- Strengthening Regulatory Coordination: Emphasis on country-specific regulatory bodies to harmonize regulations and streamline trials with AMA would enhance the trial process.
- Investment in Local Capacity: Focus on increasing investment in the local facilities to foster sustainability in the African clinical trial ecosystem would enhance the region’s clinical study capacity.
Download Free sample to learn more about this report.
Africa Clinical Trials Market Growth Factors
Growing Burden of Chronic Conditions and Increasing Demand for Cost-effective Products Fuels Market Growth
The growing burden of chronic conditions such as cancer, diabetes, Ebola virus, cardiovascular diseases (CVD), Parkinson’s, and others has been fueling the demand for effective treatment options.
- For instance, as per the data published by the World Heart Federation, in 2019, more than 1 billion people were living with cardiovascular disease in Africa. In the same year, around 1 million deaths in sub-Saharan Africa were due to CVD, accounting for 5.4% of global CVD deaths and 13.0% of the total deaths in Africa.
The growing burden of disease has been increasing the annual cost of the prevention, diagnosis, and treatment of the disease.
- For instance, as per the data published by Profmed, the annual cost of cancer treatment in South Africa can range between USD 542.0 and USD 24,381.0.
Such high costs are sometimes difficult for the general population to cover, especially in low- and middle-income countries, leading to limited adoption of these treatment options. To increase the adoption of effective treatments, pharmaceutical and biotechnological companies have increased their focus on developing cost-effective therapeutics for the prevention and treatment of diseases. This factor has been fueling the number of clinical studies being conducted in the region.
- For instance, as per the data published by SciDev.Net in April 2024, Africa has placed an increasing emphasis on vaccine development in recent years.
The increasing focus of pharmaceutical, biotechnology, and medical device companies on R&D has been fueling the demand for effective drug development, and, thereby, the number of clinical studies being conducted.
Other Drivers
- Emphasis on Disease Specific Trials: The focus on the development of therapeutics for highly prevalent diseases in Africa, such as malaria, HIV, Ebola, and others, has also been fueling the number of trials being conducted in the region.
- Innovative Trial Designs: The use of adaptive and remote trial designs to overcome logistical challenges and increase the effectiveness of clinical research has also been positively impacting the market growth.
- Capacity Building and Training: Developing skilled personnel and modern infrastructure through initiatives from Africa CDC, AUDA-NEPAD, and private sector partnerships has been fueling the number of clinical trials being conducted in the region.
- Presence of CROs Focusing on Addressing Africa’s Potential to Overcome Global Challenges: The high burden of communicable and non-communicable diseases in the region offers a genetically diverse pool of patient population for conducting clinical studies. CRO companies such as FHI Clinical have been focusing on using this as an opportunity to conduct clinical studies for effective therapeutic development.
RESTRAINING FACTORS
Challenges Associated with Conducting Clinical Studies in African Countries Restricts Market Growth
The low cost of conducting research studies in Africa has been fueling the number of trials conducted in the region. However, challenges such as lack of financial capacity and limited human resources, among others, have been limiting the number of trials conducted in the region.
- For instance, as per the data published by BioMed Central Ltd in 2020, while conducting a clinical trial in Malawi, the researchers faced delays in getting regulatory approvals from international institutes and ethical approvals from Malawi. The researchers had to put extra time and effort into the same.
- In the same study, the enrollment process was very slow. The subjects who were enrolled based on the inclusions and exclusions were not able to survive the study period due to poor health.
Such challenges faced while conducting trials in Africa limit the market players from registering trials in the region, thereby restricting Africa clinical trials market growth.
CHALLENGING FACTORS
- Infrastructure and Capacity Constraints: Limited development in the healthcare infrastructure, particularly in rural areas, hinders trial scalability and participant retention in the region.
- Regulatory Barriers: Variability in national regulations complicates trial approval processes. However, the AMA has been emphasizing streamlining regulations across countries.
- Funding and Resource Limitations: Limited funding for research studies, along with many countries in the region relying on foreign aid, has been affecting the quality and continuity of research.
- Ethical and Community Engagement Issues: Ethical concerns about informed consent and a lack of community engagement pose significant challenges, especially in trials involving experimental treatments.
Africa Clinical Trials Market Segmentation Analysis
By Phase Analysis
Increasing Focus of Academic and Research Institutes on Conducting Research Studies in Region is Responsible for Phase III Segment’s Dominance
By phase, the market is segmented into phase I, phase II, phase III, and phase IV.
The phase III segment dominated the market in 2023. The segment’s dominance in the market is attributed to the increasing focus of academic and research institutes worldwide on conducting trials in Africa.
- For instance, in November 2020, Serum Institute of India Pvt. Ltd. initiated a phase III clinical trial to study the safety and efficacy of VPM1002 in preventing tuberculosis in infants. The study is being conducted at 12 locations in Africa, including South Africa, Kenya, Tanzania, Uganda, and Gabon. The expected completion date of the study is December 2025.
The phase II segment is expected to grow at the fastest CAGR during the forecast period. The segment’s growth is attributed to increasing demand for effective drug development. Moreover, the increasing emphasis of the pharmaceutical, biotechnology, and medical devices companies on conducting trials in the region at a cheaper cost as compared to the U.S. and European countries such as the U.K. and Germany has also been fueling the segment’s growth.
To know how our report can help streamline your business, Speak to Analyst
By Application Analysis
Increasing Focus of Market Players on Expansion of Application of its Products for Clinical Trials Fuels Infectious Disease Segment’s Dominance
On the basis of application, the Africa clinical trials market is segmented into oncology, CNS disorder, cardiology, infectious disease, metabolic disorder, renal/nephrology, and others.
The infectious disease segment dominated the market in 2023, accounting for the maximum Africa clinical trials market share. The growing burden of infectious diseases, such as Ebola virus, hepatitis, chikungunya, and others, in Africa has been fueling the demand for effective therapeutics. In order to fulfill this demand, market players have been focusing on conducting trials in the region for effective therapeutic development.
- For instance, as per the data published by the Lance, since 1976 Democratic Republic of Congo has experienced 14 Ebola Virus Disease (EVD) outbreaks, with at least one outbreak each year from 2017 to 2022.
Moreover, the oncology segment is expected to grow at the fastest CAGR during the forecast period. The segment’s dominance is attributed to the presence of a large patient population which helps in increasing the efficiency of the clinical trials.
REGIONAL INSIGHTS
Based on geography, the market is studied across West, Central, East, and South Africa.
The market in South Africa accounted for the maximum share of the total Africa market in 2023, generating a revenue of USD 0.56 billion. The market’s growth in the sub-region is attributed to the strong presence of Contract Research Organizations (CROs) such as Medpace, Laboratory Corporation of America Holdings, IQVIA Inc., Syneos Health, and others, which has been fueling the outsourcing of clinical studies by small and mid-sized pharmaceutical companies.
The market in East Africa is expected to grow at a significant CAGR during the forecast period. The market’s growth in the region is attributed to the strong regulatory framework in the countries of East Africa, which has been attracting pharmaceutical companies and Contract Research Organizations (CROs) to conduct trials in the region.
- For instance, in Kenya, clinical trials are regulated and guided by two regulatory bodies, including the Pharmacy and Poisons Board (PPB) and the National Commission for Science, Technology and Innovation (NACOSTI).
Moreover, the markets in West and Central Africa are expected to grow throughout the forecast period due to the increasing number of clinical trials being conducted in the regions.
- For instance, as per the data published by the World Health Organization (WHO), in 2022, 40 clinical trials were registered in Ghana, whereas in 2016, only 19 clinical trials were registered in the country.
KEY INDUSTRY PLAYERS
Strong Emphasis of Market Players on Partnerships and Collaborations to Strengthen their Product Offerings
Market players such as IQVIA Inc., Laboratory Corporation of America Holdings, ICON plc, Syneos Health, and Charles River Laboratories are among the major players, accounting for a significant portion of the Africa clinical trials market value. The significant presence of these companies in the market is due to their focus on new services and platform launches to increase the efficiency of their offerings.
- For instance, in June 2024, IQVIA Inc. announced the launch of One Home for Sites. This platform consolidates multiple software applications and portals related to clinical trials into a single dashboard and sign-on. This helps clinical research sites manage their tasks and systems across all their trials effectively.
Moreover, pharmaceutical, biotechnological, and medical device companies such as Pfizer Inc., Novartis AG, and F. Hoffmann-La Roche Ltd, among others, have been focusing on conducting clinical studies in Africa.
- Similarly, Antiva Biosciences initiated a phase II clinical trial in November 2019 to study the safety and efficacy of Olaparib (MK-7339) in combination with Pembrolizumab (MK-3475) for the treatment of homologous recombination repair mutation (HRRm) and homologous recombination deficiency (HRD)-positive advanced cancer. The expected completion date of the study is in July 2025. The study was conducted at 135 locations which included South Africa.
List of Top Africa Clinical Trials Companies:
- IQVIA Inc. (U.S.)
- ICON plc (Ireland)
- Syneos Health (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- Medpace Holdings, Inc. (U.S.)
- Charles River Laboratories (U.S.)
- Novotech (Australia)
- Parexel International Corporation (U.S.)
- Pharmalys (U.K.)
KEY INDUSTRY DEVELOPMENTS:
- September 2024 – Medpace expanded its rare disease and metabolic leadership team with the addition of Dr. Loren Peña.
- January 2024 – Laboratory Corporation of America Holdings collaborated with Hawthorne Effect, Inc., to advance decentralized clinical trial capabilities for pharma, biotech, and medical device sponsors.
- October 2023 – IQVIA Inc. collaborated with Argenx SE to advance treatment to patients with rare autoimmune diseases through innovative and integrated technology-enabled pharmacovigilance (PV) safety services and solutions.
- June 2023 – Laboratory Corporation of America Holdings announced the formation of a new company, Fortrea, after spinning-off Labcorp's Clinical Development and Commercialization Services business. This initiative allowed the company to capture the enormous opportunity independently.
- June 2023 – ICON plc launched the latest version of the digital platform to support seamless integration of ICON patient, site, and sponsor services. This platform enabled customization for any therapeutic area and study design, from traditional to fully decentralized.
REPORT COVERAGE
The Africa clinical trials market report provides a detailed competitive landscape and market insights. The report includes key insights, such as top industry developments covering partnerships, mergers, and acquisitions as well. Additionally, it focuses on key points, such as new solution launches in the market. Furthermore, the report covers regional analysis of different market segments, profiles of key market players, market trends, and the impact of COVID-19 on the market. The report consists of quantitative and qualitative insights that have contributed to the market's growth.
Request for Customization to gain extensive market insights.
Report Scope & Segmentation
|
ATTRIBUTE |
DETAILS |
|
Study Period |
2019-2032 |
|
Base Year |
2023 |
|
Estimated Year |
2024 |
|
Forecast Period |
2024-2032 |
|
Historical Period |
2019-2022 |
|
Growth Rate |
CAGR of 7.2% from 2024-2032 |
|
Unit |
Value (USD billion) |
|
Segmentation |
By Phase
|
|
By Application
|
|
|
By Region
|
Frequently Asked Questions
Fortune Business Insights says that the Africa market stood at USD 0.91 billion in 2023 and is projected to record a valuation of USD 1.68 billion by 2032.
In 2023, the South African market value stood at USD 0.56 billion.
The market is predicted to exhibit a CAGR of 7.2% during the forecast period.
By phase the phase III segment led the market in 2023.
Increasing demand for the development of cost-effective treatments and the increasing number of clinical trials being conducted in Africa have been fueling the market’s growth.
IQVIA Inc., Laboratory Corporation of America Holdings, ICON plc, Syneos Health, and Charles River Laboratories are the top players operating in the market.
Related Reports
-
US +1 833 909 2966 ( Toll Free )
-
Get In Touch With Us


