Autism: Pipeline Review, 2024
KEY MARKET INSIGHTS
Autism, now also referred to as autism spectrum disorder, refers to a diverse group of neurodevelopmental disorders that is generally diagnosed during the individual’s adolescent years. Autism particularly relates to the brain development and impacts how an individual interacts or communicates in a social situation. The phrase of spectrum in the ‘autism spectrum disorder’, reflects the diverse range of symptoms and their severity. Some of the disorders that comes under autism spectrum disorder includes:
- Autistic Disorder
- Asperger's Syndrome
- Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS)
- Others
Epidemiology:
Several recent studies have strongly indicated that the prevalence of autism is rising rapidly, however, there is a lack of consensus in terms of understanding what causes this diseases. For instance, certain studies have indicated that genetic influences and environmental factors which includes social determinants such as limited healthcare insurance coverage, and lower socioeconomic status may cause this condition. According to the data published by the World Health Organization (WHO) in November 2023, an estimated 1 in 100 children suffer from this disease globally. Also, the WHO goes on to reiterate that the reported prevalence of this disease vastly differs across various geographies.
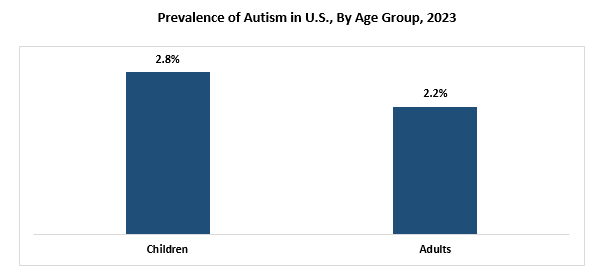
According to the data published by Centers for Disease Control and Prevention (CDC) in 2023, the prevalence of autism has increased in the U.S. Currently, the prevalence of autism in the U.S. is 1 in 36 children, which is considerably higher than the previously reported prevalence of 1 in 44 children in the U.S. Furthermore, the CDC also states that in the U.S., 4 in 100 boys and 1 in 100 girls suffer from this condition.
Therapeutic Assessment:
Diagnosis:
According to the Centers for Disease Control and Prevention (CDC) of the U.S., the diagnosis of this condition relies on two sources of information: the parents’ or the child’s caregiver’s description of the child’s development or the professional’s assessment of the child’s behaviour. The CDC also states that the American Psychiatric Association's Diagnostic and Statistical Manual, Fifth Edition (DSM-5) gives a standardized criteria for the diagnosis of autism spectrum disorder. In terms of the DSM-5’s criteria for autism diagnosis, the child must have persistent deficits in each of the three areas of social communication and interaction. Similarly in Europe, the diagnosis of autism spectrum disorder generally entails the involvement of a multidisciplinary team of psychiatrists, speech therapists and psychologists. Even in Europe, the criteria for diagnosis of autism is standardized with adherence to guidelines published by Diagnostic and Statistical Manual of Mental Disorders (DSM-5) or the International Classification of Diseases (ICD-11).
Treatment:
In terms of the treatment of autism, often a multi-pronged treatment approach is considered. This includes the utilization of communication & behavioural therapies and the drug therapies. Several research studies have demonstrated the strong efficacy of the adoption of combination therapies for autism. Some of the communication & behavioural therapies utilized for the autism spectrum disorder includes speech & language therapy, applied behavioral analysis (ABA), occupational therapy, cognitive behavioral therapy (CBT), and others. In terms of drug therapies, as of now, no drug has been approved to treat or cure autism. However, certain medications are utilized to help the healthcare professionals manage the symptoms of this condition such as irritability, insomnia, depression, and lack of focus. Some of these mentioned drug therapies have received regulatory approvals from agencies from the U.S. FDA, while the others are used off-label.
Key Products:
The drugs that have received approval from the U.S. FDA for autism spectrum disorder treatment are risperidone, and aripiprazole. While risperidone can be prescribed for children between the ages of 5 to 16 years to help with aggression and irritability, aripiprazole can be given to children between the ages of 6 to 17 years. However, in Europe, there are no approved medications for the treatment of autism symptoms, while certain guidelines support their utilization.
Major Players in Autism:
Some of the major players in the autism market are Janssen Global Services, LLC, Amneal Pharmaceuticals LLC, Bristol-Myers Squibb Company, Aurobindo Pharma, and other companies.
Autism Spectrum Disorder Treatment Market Overview:
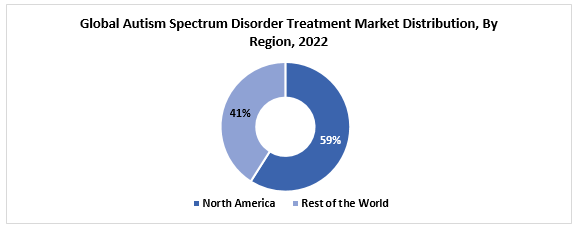
The global autism spectrum disorder treatment market size was valued at USD 6.94 billion in 2022. The market is projected to grow from USD 7.41 billion in 2023 to USD 13.14 billion by 2030, exhibiting a CAGR of 8.5% during 2023-2030. North America region is the most dominant region in the global market. Some of the factors which contribute to the growth of the global market is the presence of potential pipeline candidates that may be approved, the strong sustained increase of autism prevalence, and the boost in the awareness amongst the general population.
.png)
Pipeline Analysis:
Overview of the Pipeline:
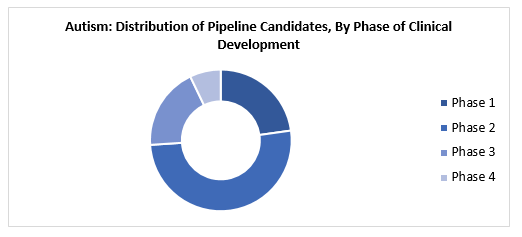
At present around 75% of the pipeline candidates for autism are in the phase-1 and phase-2 stages of clinical trials combined. Majority of the studies are sponsored by industry and governmental institutions.
Pipeline By Mechanism of Action:
The mechanism of action of the earliest therapies that have gained regulatory approvals for autism such as risperidone, and aripiprazole are the blockade of serotonin 2A and dopamine D2 receptors for risperidone. For aripiprazole, the mechanism of action is the stimulation and the inhibition of dopamine as it engages the D2 receptor. In terms of research activity, several players have focused on the genetic mechanisms influencing autism. In recent times, some of the companies have focused on the following mechanism of actions:
- Catecholamines Modulation: Catecholamines are a set of neurotransmitters that is supplied to almost all tissues of the body, and almost all tracts of the brain, hence this is a common neurologic regulatory mechanism for all symptoms of autism. The pipeline drugs belonging to this class of mechanism of action, may release a presynaptic mechanism that inhibits the tyrosine hydroxylase, and eventually the synthesis, storage and release of all catecholamines. This may regulate the catecholamines by bringing it back to a homeostatic balance.
- Galectin-3 Inhibition: Several research studies have noted that the levels of protein galectin-3 are considerably higher in children with ASD. Hence, the inhibition of this protein may enable the control of the autism symptoms.
- Accelerated Protein Digestion and Amino Acid Absorption: The drugs belonging to this mechanism of action, emphasize on the role of amino acids in neurological disorders. This is because, amino acids are a key player in terms of new neurotransmitter formations, the regulation of CNS genes, and also the synthesis of proteins. These drugs play a key role in the formation of enzymes that leads to a balanced amino acids pool. The studies related to this pipeline candidate have indicated enzymatic deficiencies play a role in autism.
Pipeline By Route of Administration:
In terms of the route of administration of the pipeline drugs, a significant number of these drugs under clinical trials for autism treatment, have the oral route of administration. This is because, as a number of the patients of autism are adolescents, the oral form of medication administration will be easier for them, and will make them more treatment adherent as they can be administered by their parents or caregivers at the homecare settings.
Pipeline, By Molecule Type:
The majority of the players engaged in the clinical trials for autism treatment have focused on the development of the small molecule drugs. Some of the players with small molecule drugs in their product pipelines for autism includes the emerging company of Axial Therapeutics.
Pipeline, By Company:
In terms of pipeline drugs for autism treatment, the number of these drugs in the advanced stages of clinical studies are limited. A number of the companies with pipeline candidates for autism treatment, are emerging biopharmaceutical companies that based in the U.S. Some of the companies with pipeline drugs are Yamo Pharmaceuticals, Curemark, and Astrogen, Inc.
Clinical Trial Insights:
Ongoing Clinical Trials: Some of the key pipeline candidates under clinical trials are as follows:
Phase 1:
- STP1: Stalicla SA
- Study Description: The main purpose of this study is to evaluate the safety and tolerability, pharmacokinetics and pharmacodynamics, as well as exploratory efficacy of the pipeline candidate of STP1 in a subgroup of patients suffering from autism spectrum disorder (ASD).
- SB-121: Scioto Biosciences, Inc.
- Study Description: This study is a multi-dose, randomized, double-blind, placebo-controlled, crossover study assessing the safety and tolerability of multiple doses of SB-121 over a 28 day period for a group of subjects in the age group of 15 to 45 years, diagnosed with autistic disorder.
Phase 2:
- ML-004: MapLight Therapeutics
- Study Description: This study model is a multi-center, randomized, double-blind, parallel-group, placebo-controlled study that will recruit approximately 150 adolescents and adult subjects suffering from ASD. The main purpose of this clinical trial is to study the efficacy of this pipeline drug when compared with placebo in terms of the improvement of social communication deficits in patients suffering from ASD. The study type of this clinical trial is interventional, with treatment being the primary purpose. The masking in this study is quadruple, with the interventional model being parallel assignment. The allocation for this clinical trial is randomized.
- ARD-501: Aardvark Therapeutics, Inc.
- Study Description: This study is a blinded, placebo controlled, cross-over trial that evaluates the safety of the two dose-levels of the pipeline candidate of ARD-501 in patients suffering from ASD. The study type of the clinical trial is interventional, and the primary purpose is treatment. The allocation is randomized for this study, and the interventional model is crossover assignment. The masking for this study is double.
Phase 3:
- CM-AT: Curemark
- Study Description: This study is an open-label extension study of CM-AT for the treatment of children suffering from autism with all the levels of fecal chymotrypsin. CM-AT is a proprietary enzyme that shall be administered to children suffering from this disorder, thrice a day. The study type of this clinical trial is interventional, with the primary purpose being treatment. The interventional model is single group assignment and the masking is none (open label).
- AZD0901: Astrogen, Inc.
- Study Description: This study aims to demonstrate the efficacy and safety of AST-001, when compared to placebo, in terms of the improvement of the core symptoms of autism spectrum disorder (ASD) in children. For this study, the study type is interventional and the primary purpose is treatment. The allocation for this clinical trial is randomized, and the interventional model is parallel assignment. The masking for this clinical trial is quadruple.
Future Trial Prospects:
- January 2024: The Israel based pharmaceutical company of Sci Sparc announced the beginning of clinical trial to compare the effects of SCI-210 therapy with the standard CBD treatment for the management of ASD symptoms.
- November 2021: Axial Therapeutics announced the dosing of the first subjects in the global Phase 2b clinical trial for the irritability associated with the autism spectrum disorder (ASD).
Regulatory Landscape:
- FDA and EMA Approvals: In terms of regulatory agencies, the U.S. FDA and EMA are the key agencies. Till date, the U.S. FDA has granted the approval to 2 drugs for the management of autism symptoms, and the EMA has granted no approvals till date. However, to reduce this limitation of no approvals till date, there has the formation of consortiums such as the AIMS-2-TRIALS consortium.
- Orphan Drug Designations: The number of drugs receiving the regulatory designation from the U.S. FDA of an Orphan Drug Designation (ODD) has been comparatively limited. However, in February 2023, the U.S. FDA granted an ODD to the drug KETARX (ketamine), which is currently in Phase 2 clinical trials for the treatment of Rett Syndrome, which is considered to be a disease on the autism spectrum.
- Market Authorization Challenges: Some of the market authorization challenges faced by the autism pipeline candidates includes a lack of regulatory approvals, and the skepticism in terms of their ability to treat the core symptoms of the disease.
Report Scope
- A thorough assessment of the pipeline products by areas such as development stage; route of administration; drug class; indication; sponsor; molecule type and drug target
- Comprehensive profiles of the pipeline products with details such as company overview; product description; R&D status; development activities; mechanism of action; molecule type; development stage; indications; funding and route of administration
- Overview of dormant and discontinued pipeline products
- Key insights about the epidemiology of conditions being treated by the pipeline products and overview of the addressable or current market for the pipeline products
- Overview of the latest developments; news articles, press releases, and relevant conferences
Report Methodology
- All pipeline reports are built through the analysis of data primarily collected through credible desk research sources. Secondary research is supplemented by interviews conducted with key opinion leaders.
- Desk research sources include global and regional clinical trials databases; annual reports, websites, press releases & investor presentations of companies; white papers; news articles; reports published by industry associations; articles/reports published on databases such as NCBI, ResearchGate; internal databases
Reasons to Buy this Report
- Develop effective growth strategies based on a comprehensive overview of the R&D activity and pipeline products for Autism
- Identify emerging players or competition in the market based on pipeline products and develop strategies to counter the emergence of these players
- Identify the focus of leading players about R&D for Autism
- Identify potential companies from a partnership or acquisition point of view based on current synergy in R&D activities or strategies to diversify R&D focus to drive growth in business
- Analyze the reasons behind dormant and discontinued products to make changes in the R&D focus if necessary
-
US +1 833 909 2966 ( Toll Free )
-
Get In Touch With Us

