Exocrine Pancreatic Insufficiency Therapeutics Market Size, Share & Industry Analysis, By Therapy (Pancreatic Enzyme Replacement Therapy (PERT), Nutritional Therapy), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies & Drug Stores, Others) and Regional Forecast, 2026-2034
KEY MARKET INSIGHTS
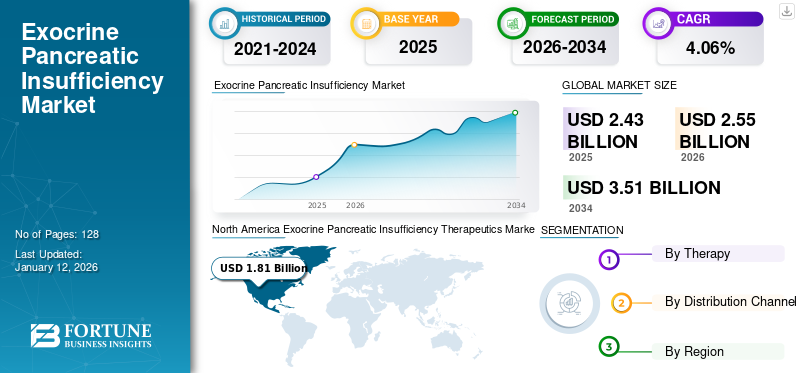
The global exocrine pancreatic insufficiency therapeutics market size stood at USD 2.43 billion in 2025. It is projected to grow from USD 2.55 billion in 2026 to USD 3.51 billion by 2034, exhibiting a CAGR of 4.06% during the forecast period. North America dominated the exocrine pancreatic insufficiency (EPI) therapeutics market, with a market share of 3.85% in 2025. The increasing prevalence of disorders associated with exocrine pancreatic insufficiency will propel the demand for advanced therapeutics.
Exocrine pancreatic insufficiency is a medical condition characterized by a deficiency of the exocrine pancreatic enzymes. This deficiency results in maldigestion or the inability to properly digest food. The diagnosis of exocrine pancreatic insufficiency is often purely clinical because the signs and symptoms of the disease often go undetected. This disease can often only be detected by various diagnostic tests, such as blood, stool, malabsorption, and pancreatic function tests.
In the current scenario, several positive factors are providing major impetus to the exocrine pancreatic insufficiency therapeutics market trends. There is an increased awareness with respect to this disorder and the possible repercussions or negative impact if it is neglected. A number of chronic and serious disorders, such as cystic fibrosis, celiac disease, and tumors, are associated with exocrine pancreatic insufficiency.
Download Free sample to learn more about this report.
One of the critical factors contributing to the growth of the global market is that several acute and chronic pancreatic disorders often lead to exocrine pancreatic insufficiency. Improved understanding of exocrine pancreatic insufficiency have contributed substantially to the introduction of new therapeutic measures and continued R&D by key market players. While the prevalence of exocrine pancreatic insufficiency in the general population is difficult to gauge, a number of key gastrointestinal disorders are linked to this disease. This is estimated to severely increase its prevalence in the global scenario, augmenting the exocrine pancreatic insufficiency therapeutics market growth.
Global Exocrine Pancreatic Insufficiency Therapeutics Market Overview
Market Size & Forecast:
- 2025 Market Size: USD 2.43 billion
- 2026 Market Size: USD 2.55 billion
- 2034 Forecast Market Size: USD 3.51 billion
- CAGR: 4.06% from 2026–2034
Market Share:
- North America dominated the exocrine pancreatic insufficiency (EPI) therapeutics market with a 3.85% share in 2025, driven by high awareness of gastrointestinal disorders, rapid adoption of advanced therapeutics, and strong R&D presence.
- Pancreatic Enzyme Replacement Therapy (PERT) is expected to hold the largest market share and grow at the highest CAGR due to its established role as the conventional and most effective treatment, projected to generate USD 2.12 billion in revenue by 2025.
Key Country Highlights:
- Japan: Among the few countries providing detailed prevalence data for EPI; expected to reach USD 82.3 million by 2025, with growth supported by early adoption of advanced gastrointestinal therapeutics.
- United States: Acute pancreatitis is one of the leading gastrointestinal causes of hospitalization, significantly contributing to EPI prevalence; high treatment penetration and strong pharmaceutical pipelines drive demand.
- China: Projected to grow at a CAGR of 9.90% during the forecast period due to rising awareness, increasing disposable income, and growing adoption of advanced therapies.
- Europe: Expected to grow at a CAGR of 7.0% as under-penetration and rising awareness in high-growth countries such as the U.K., France, and Germany open opportunities for market expansion.
MARKET DRIVERS
“Increased Awareness and Innovation of Therapeutics is Likely to Propel the Pancreatic Exocrine Insufficiency Treatment Market”
One of the crucial drivers for the global EPI therapeutics market growth is the increased awareness regarding exocrine pancreatic insufficiency and the corresponding effect on the overall health of the individual. For instance, acute pancreatitis is one of the leading gastrointestinal causes of hospital admission in the U.S. and causes a high prevalence of exocrine pancreatic insufficiency. Trends such as these are anticipated to drive the growth of the exocrine pancreatic insufficiency therapeutics market. For instance, in October 2019, AzurRx BioPharma announced that the first patients were dosed of its drug candidate MS1819-SD for exocrine pancreatic insufficiency.
“Increasing Prevalence of Associated Disorders to Drive the Market”
Other critical driving factors are the rising prevalence of associated disorders, leading to the high prevalence of exocrine pancreatic insufficiency. Exocrine pancreatic insufficiency therapeutics have undergone major R&D innovations since the time they were initially introduced to the general public. A number of different clinical conditions of acute and serious nature have a high prevalence of exocrine pancreatic insufficiency. These include acute and autoimmune pancreatitis, cystic fibrosis, Shwachman–Diamond syndrome, and pancreatic tumors. These factors have led to the development of new therapeutics that have several advantages for the effective management of the disorder. Another key driving factor leading to the wide adoption of these therapies, such as pancreatic enzyme replacement therapy (PERT) for efficient treatment, is the increasing awareness resulting from the increasing prevalence of the disorder.
SEGMENTATION
By Therapy Analysis
“The Pancreatic Enzyme Replacement Therapy (PERT) Segment to Generate Highest Growth Rate During the Forecast Period”
The global market can be segmented by therapy into pancreatic enzyme replacement therapy (PERT) and nutritional therapy.
The PERT segment is estimated to hold the dominant share of the exocrine pancreatic insufficiency therapeutics market owing to their status as the conventional therapy. This is expected to enable pancreatic enzyme replacement therapy to grow at the highest CAGR due to its positive features, such as efficiency, efficacy, and potency in terms of treatment.
- By therapy, the pancreatic enzyme replacement therapy (PERT) segment is projected to generate USD 2.12 billion in revenue by 2025.
To know how our report can help streamline your business, Speak to Analyst
Nutritional therapy is also one of the critical components of the treatment regime for exocrine pancreatic insufficiency. This segment will hold a lower market share and will grow at a lower CAGR, but its importance cannot be undermined. This is because poor nutrition is expected to result in more complications and a higher mortality.
By Distribution Channel Analysis
“Hospital Pharmacies Segment Likely to Hold the Highest Share among Distribution Channels.”
Based on distribution channel, the market for exocrine pancreatic insufficiency therapeutics can be segmented into hospital pharmacies, retail pharmacies & drug stores, and others. The hospital pharmacies segment is anticipated to have the dominant share because medical professionals at these institutions often prescribe these medications after due diagnostic measures.
- By distribution channel, the hospitals pharmacies segment is expected to hold a 67.9% share in 2025.
The retail pharmacies and drug stores segment is anticipated to hold the second-largest share because these institutions can provide more efficient care when refilling prescriptions. The segment is also anticipated to experience opportunities for revenue increase due to the increasing usage of online pharmacy facilities, which are often supported by these institutions.
REGIONAL ANALYSIS
North America Exocrine Pancreatic Insufficiency Therapeutics Market Size, 2024 USD Billion
To get more information on the regional analysis of this market, Download Free sample
North America dominated the market with a valuation of USD 1.89 billion in 2025 and USD 1.98 billion in 2026. The rapid adoption of advanced therapeutics coupled with the usage of consistent and continued R&D across the region will drive the market growth in the region. Besides this, the increasing prevalence, coupled with the high awareness of associated gastrointestinal disorders, is expected to fuel the growth of the market in North America during the forecast period. The U.S. market is projected to reach USD 1.69 billion by 2026.
Asia Pacific is expected to witness the highest growth in terms of market value. Owing to the increasing disposable incomes across the region, there is a greater adoption of advanced therapeutics with respect to critical gastrointestinal disorders and its impact on overall health. For instance, Japan is one of the key countries across the world apart from North America and Europe to give population distributions of the disorder. This would drive the market growth across the region.
The Japan market is projected to reach USD 0.09 billion by 2026, the China market is projected to reach USD 0.06 billion by 2026, and the India market is projected to reach USD 0.03 billion by 2026.
On the other hand, Europe is projected to witness significant growth in the market due to the under-penetration of therapeutics. The high-growth countries, including the U.K., France, and Germany, would contribute to the market growth in Europe. The rest of the world market for exocrine pancreatic insufficiency therapeutics is anticipated to undergo comparatively higher growth than the other key regions due to the under-penetration of the market, where the revenue growth has not reached its complete potential and future growth prospects.
The UK market is projected to reach USD 0.05 billion by 2026, and the Germany market is projected to reach USD 0.07 billion by 2026.
INDUSTRY KEY PLAYERS
“Market Players like AbbVie, and Allergan Likely to Strengthen the Market Position across the Globe”
Some key companies dominate the exocrine pancreatic insufficiency therapeutics market due to their strong product portfolio, key strategic decisions and dominance of market share. These includes a group of 2-3 key companies with wider geographic presence. But some new market players with new R&D is anticipated to result in strong regulatory approvals despite some recent setbacks in R&D. For instance, in April 2016, Anthera Pharmaceuticals announced that its product candidate Sollpura, a novel, non-porcine pancreatic enzyme replacement therapy failed its exocrine pancreatic insufficiency trial.
List Of key Companies Covered
- AbbVie Inc.
- Janssen Pharmaceuticals, Inc.
- Allergan
- AzurRx
- CHIESI Farmaceutici S.p.A.
- Other Prominent Players
INDUSTRY DEVELOPMENT:
- February 19, 2024: AbbVie presented new data from its gastroenterology portfolio at the 19th Congress of the European Crohn's and Colitis Organisation (ECCO). While the focus was primarily on inflammatory bowel diseases, AbbVie continues to support research in gastrointestinal disorders, including EPI.
REPORT COVERAGE
The increased awareness of exocrine pancreatic insufficiency and anticipated product launches based on new R&D is expected to fuel the exocrine pancreatic insufficiency therapeutics market growth.
The report also provides an elaborate analysis of the global market dynamics and competitive landscape. Various key insights presented in the report are the prevalence of major disorders associated with exocrine pancreatic insufficiency, pipeline analysis, recent industry developments such as mergers & acquisitions, regulatory scenarios in crucial countries, and new product launches. Along with this, other key insights include overview of new R&D in exocrine pancreatic insufficiency treatment, key strategies adopted by market leaders, competitive landscape, and company profiles.
Request for Customization to gain extensive market insights.
Report Scope & Segmentation
|
ATTRIBUTE |
DETAILS |
|
Study Period |
2021-2034 |
|
Base Year |
2025 |
|
Forecast Period |
2026-2034 |
|
Historical Period |
2021-2024 |
|
Unit |
Value (USD billion) |
|
Segmentation |
By Therapy
|
|
By Distribution Channel
|
|
|
By Geography
|
Frequently Asked Questions
According to Fortune Business Insights, the global exocrine pancreatic insufficiency (EPI) therapeutics market was valued at USD 2.55 billion in 2026 and is projected to reach USD 3.51 billion by 2034, driven by rising awareness, improved diagnostics, and the increasing adoption of enzyme replacement therapies.
Pancreatic enzyme replacement therapy (PERT) leads the EPI therapeutics market due to its effectiveness in managing enzyme deficiencies and its wide availability under brand names like Creon, Zenpep, and Pancreaze.
Growing at a CAGR of 4.06%, the market will exhibit steady growth in the forecast period (2026-2034)
Pancreatic Enzyme Replacement Therapy (PERT) segment is expected to be the leading segment in this market during the forecast period.
Increased healthcare awareness and strong product launches will drive the growth of the exocrine pancreatic insufficiency therapeutics market.
AbbVie and Allergan are the top players in the market.
North America is expected to hold the highest market share in the market.
Increased awareness, increasing R&D, increased prevalence of associated disorders and new product launches would drive the adoption of exocrine pancreatic insufficiency therapeutics.
Notable trends include non-porcine enzyme development, integration of nutrition-based adjunct therapies, and the growth of online pharmacies as a key distribution channel for chronic disease medications.
Related Reports
-
US +1 833 909 2966 ( Toll Free )
-
Get In Touch With Us